Home / Publications / Reviews
Fathi Vavsari, V.; Shakeri P.; Balalaie S.
As one of the most important building blocks in organic synthesis, isocyanides come in for a wide range of transformations owing mostly to their unusual terminal carbon center adsorbed electrophiles, reacted with nucleophiles, get involved in radical reactions and coordinated with metal centers. The distinctive feature of isocyanide is its ready willingness to participate in multicomponent reactions (MCRs). MCRs represent a great tool in organic synthesis for the construction of new lead structures in a single procedure introducing both structural diversity and molecular complexity in only one step. Isocyanide-based multicomponent reactions (IMCRs) have become powerful approach for the synthesis of complex molecules providing high degree of atom and bond economy under very mild reaction conditions. The use of enantiomerically pure isocyanides can, in principle, bring about two advantages: (i) the possibility to obtain a stereochemically diverse adduct, controlling the absolute configuration of the starting isocyanide; and (ii) the possibility to induce diastereoselection in the multicomponent reaction. The most commonly-used IMCRs are the Ugi and Passerini reactions. Many published reviews have focused on the Ugi and Passerini reactions from different viewpoints, but this review describes advances in the application of chiral isocyanides in MCRs. The rational for applying such diversity generating chemistries is also discussed.
Hamdan, F.; Tahoori, F.; Balalaie, S.
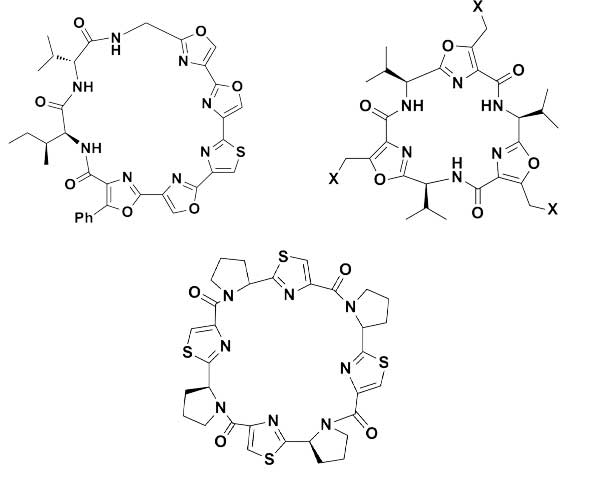
Cyclopeptides can be considered as naturally biologically active compounds. Over the last several decades, many attempts have been made to synthesize complex naturally occurring cyclopeptides, and great progress has been achieved to advance the field of total synthesis. Moreover, cyclopeptides containing heterocyclic skeletons have been recently developed into powerful reactions and approaches. This review aims to highlight recent advances in the synthesis of cyclopeptides containing heterocyclic skeletons such as triazole, oxazole, thiazole, and tetrazole.

Fathi Vavsari, V.; Balalaie S.
Cascade reactions, as an attractive branch of organic chemistry, have been the subject of intense research in recent years. In these reactions numerous transformations occur in a single sequence, and the subsequent reactions are formed as a consequence of the functionality of the previous step. Cascade reactions are environmentally benign processes due to their one-pot procedure which accordingly proceed in a single reaction solvent, work-up, and purification step. Therefore, they are often associated with cost savings in terms of solvents, catalysts, and reagents in addition to time and effort. In recent years, cascade reactions are used for construction of complicated molecular scaffolds, especially heterocyclic natural products. Different naturally occurring compounds were synthesized through cascade (tandem) reactions; this review has been focused on the use of cascade sequences for the synthesis of heterocyclic natural products.
Fathi Vavsari, V.; Balalaie, S.
This minireview focuses on the recent advances in green synthesis of chromone derivatives which have been published over the last 5–10 years.